Order of a Reaction
Order of a Reaction: Overview
This topic covers concepts, such as, Order of a Reaction etc.
Important Questions on Order of a Reaction
For the following elementary reaction, determine its order of reaction and the dimensions of the rate constant:
For a reaction, , the rate is given by , hence, the order of the reaction is:
Order of a reaction is the sum of exponents of _____ in the rate equation.
Order of a reaction with rate constant is :
Rate constant and rate of a reaction have the same units for reactions of _____ order.
What is the order for the following reactions?
, rate
The rate law for the gas-phase reaction is rate What is the order of the reaction with respect to each of the reactants and what is the overall order of the reaction?
Explain the term order of a reaction with examples.
The following results have been obtained during the kinetic studies of the reaction:
| Experiment | Initial rate of formation of | ||
| I | |||
| II | |||
| III | |||
| IV |
Determine the rate law and the rate constant for the reaction.
In a reaction between and , the initial rate of reaction was measured for different initial concentration of and as given below:
What is the order of the reaction with respect to and ?
Suppose in a chemical reaction , it is found that the rate of the reaction doubles when the concentration of is increased four times. The order of the reaction with respect to is
It is true that :
(1) A zero order reaction is a single step reaction.
(2) A second order reaction is always a multistep reaction.
(3) A first order reaction is always a single step reaction.
(4) A zero order reaction is a multistep reaction.
For reaction products consider the graph
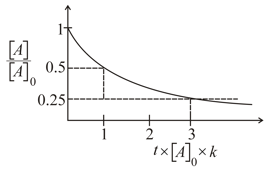
The oder of reaction is
In a reaction, the time required to complete half of the reaction was found to increase 16 times when the initial concentration of the reactant was reduced to 1/4th. What is the order of the reaction?
The rate of the reaction, Products, is given by
The order of reaction is
What is the order of a reaction which has a rate expression
The rate of the reaction,
is given by the rate expression :
If is taken in excess, the order of the reaction would be :
If a certain reaction is first order with respect to second order with respect to and zero order with respect to then what is the order of reaction?
For the following elementary reaction, determine its order of reaction and the dimensions of the rate constant:
